What Property of Caffeine Makes It Easy to Extract edu
As stated in Chapter 1, caffeine is the most widely used central nervous system (CNS) stimulant in the world. It has numerous pharmacological and physiological effects, including cardiovascular, respiratory, renal, and smooth muscle effects, as well as effects on mood, memory, alertness, and physical and cognitive performance. This chapter provides a brief summary of the metabolism and physiological effects of caffeine
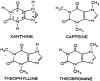
Caffeine (1,3,7-trimethylxanthine) is a plant alkaloid with a chemical structure of C8H10N4O2 (see Figure 2–1) and a molecular weight of 194.19. In pure form, it is a bitter white powder. Structurally, caffeine (and the other methylxanthines) resembles the purines. The mean half-life of caffeine in plasma of healthy individuals is about 5 hours. However, caffeine's elimination half-life may range between 1.5 and 9.5 hours, while the total plasma clearance rate for caffeine is estimated to be 0.078 L/h/kg (Brachtel and Richter, 1992; Busto et al., 1989). This wide range in the plasma mean half-life of caffeine is due to both innate individual variation, and a variety of physiological and environmental characteristics that influence caffeine metabolism (e.g., pregnancy, obesity, use of oral contraceptives, smoking, altitude). The pharmacological effects of caffeine are similar to those of other methylxanthines (including those found in various teas and chocolates). These effects include mild CNS stimulation and wakefulness, ability to sustain intellectual activity, and decreased reaction times.
The fatal acute oral dose of caffeine in humans is estimated to be 10–14 g (150–200 mg/kg body weight [BW]) (Hodgman, 1998). Ingestion of caffeine in doses up to 10 g has caused convulsions and vomiting with complete recovery in 6 hours (Dreisbach, 1974). Extreme side effects were observed in humans at caffeine intakes of 1 g (15 mg/kg) (Gilman et al., 1990), including restlessness, nervousness, and irritability, and progressing to delirium, emesis, neuromuscular tremors, and convulsions. Other symptoms included tachycardia and increased respiration.
ABSORPTION, DISTRIBUTION, AND METABOLISM
Caffeine is rapidly and completely absorbed in humans, with 99 percent being absorbed within 45 minutes of ingestion (Bonati et al., 1982; Liguori et al., 1997). When it is consumed in beverages (most commonly coffee, tea, or soft drinks) caffeine is absorbed rapidly from the gastrointestinal tract and distributed throughout body water. More rapid absorption can be achieved by chewing caffeine-containing gum or other preparations that allow absorption through the oral mucosa.
Peak plasma concentrations occur between 15 and 120 minutes after oral ingestion. This wide variation in time may be due to variation in gastric emptying time and the presence of other dietary constituents, such as fiber (Arnaud, 1987). Once caffeine is absorbed, there appears to be no hepatic first-pass effect (i.e., the liver does not appear to remove caffeine as it passes from the gut to the general circulation), as evidenced by the similarity in plasma concentration curves that follow its administration by either the oral or the intravenous route (Arnaud, 1993). Caffeine binds reversibly to plasma proteins, and protein-bound caffeine accounts for about 10 to 30 percent of the total plasma pool. The distribution volume within the body is 0.7 L/kg, a value suggesting that it is hydrophilic and distributes freely into the intracellular tissue water (Arnaud, 1987, 1993). However, caffeine is also sufficiently lipophilic to pass through all biological membranes and readily crosses the blood-brain barrier. Its elimination is by first-order kinetics and is adequately described by a one-compartment open model system (Bonati et al., 1982). In a study of adult men, a dose of 4 mg/kg (280 mg/70 kg human, or about 2–3 cups of coffee) had a caffeine half-life of 2.5–4.5 hours, and was not affected by age (Arnaud, 1988).
Because caffeine is readily reabsorbed by the renal tubules, once it is filtered by the glomeruli only a small percentage is excreted unchanged in the urine. Its limited appearance in urine indicates that caffeine metabolism is the rate-limiting factor in its plasma clearance (Arnaud, 1993). Caffeine metabolism occurs primarily in the liver, catalyzed by hepatic microsomal enzyme systems (Grant et al., 1987). In healthy humans, repeated caffeine ingestion does not alter its absorption or metabolism (George et al., 1986). It is metabolized in the liver to dimethylxanthines, uric acids, di- and trimethylallantoin, and uracil derivatives. In humans 3-ethyl demethylation to paraxanthine is the primary route of metabolism (Arnaud, 1987). This first metabolic step accounts for approximately 75–80 percent of caffeine metabolism and involves cytochrome P4501A2 (Arnaud, 1993). Paraxanthine is the dominant metabolite in humans, rising in plasma to concentrations 10 times those of theophylline or theobromine. Caffeine is cleared more quickly than paraxanthine, so 8 to 10 hours after caffeine ingestion, paraxanthine levels exceed caffeine levels in plasma (Arnaud, 1993).
The fact that the human body converts 70–80 percent of caffeine into paraxanthine with no apparent toxic effects following caffeine doses of 300–500 mg/day suggests that paraxanthine's toxicological potency is low. Formation of paraxanthine and its excretion in the urine appears to be the major pathway for caffeine metabolism (Stavric, 1988).
Hetzler et al. (1990) demonstrated that lipolytic effects of caffeine may be due to the action of paraxanthine rather than caffeine itself. Increasing concentration of plasma-free fatty acids following intravenous administration of caffeine was negatively correlated to plasma caffeine concentrations, and highly positively correlated to plasma paraxanthine concentrations. Paraxanthine has been found to be an equipotent adenosine antagonist to caffeine in vitro. Benowitz et al. (1995) demonstrated that both caffeine and paraxanthine significantly increased diastolic blood pressure, plasma concentrations of epinephrine, and free fatty acids. Plasma levels of caffeine peaked 75 minutes after oral dosing of caffeine, while plasma levels of paraxanthine peaked at 300 minutes after an oral dose of paraxanthine. At doses of 4 mg/kg BW, caffeine and paraxanthine were equipotent. At doses of 2 mg/kg BW, however, caffeine was more potent. Benowitz and colleagues (1995) concluded that after a single dose of caffeine, paraxanthine concentrations are relatively low and probably do not contribute much to the effect of caffeine. However, with long-term exposure to caffeine there is a substantial accumulation of paraxanthine, and thus paraxanthine almost certainly contributes to the pharmacologic activity of caffeine. It would be reasonable to expect then, that with long-term caffeine exposure, paraxanthine would also contribute to development of tolerance to caffeine and withdrawal symptoms.
There is likely to be considerable individual variation in the extent of conversion of caffeine to paraxanthine, and because paraxanthine has pharmacologic activity, the extent of conversion would be a factor in determining individual differences in response to caffeine.
FACTORS AFFECTING CAFFEINE METABOLISM
Caffeine metabolism is increased by smoking, an effect mediated by an acceleration in its demethylation (it also increases xanthine oxidase activity) (Parsons and Neims, 1978). Smoking cessation returns caffeine clearance rates to nonsmoking values (Murphy et al., 1988). A number of studies with rodents have demonstrated an additive effect of caffeine and nicotine on both schedule-controlled behavior and locomotor activity (Lee et al., 1987; Sansone et al., 1994; White, 1988). However, data in humans are scarce. Kerr et al. (1991) found both caffeine and nicotine facilitated memory and motor function in a variety of psychomotor tasks. Though there were differences across tasks, combining caffeine and nicotine did not appear to produce a greater effect than either drug alone. Conversely, nicotine did not decrease the effectiveness of caffeine.
The effects of caffeine on women have been examined in the context of its effects on menstrual function, interactions with oral contraceptives, pregnancy and fetal health, and postmenopausal health. Earlier studies suggested that elimination of caffeine may vary across the menstrual cycle, with elimination being about 25 percent longer in the luteal phase (Balogh et al., 1987). More recent studies, however, indicate no significant effects on caffeine pharmacokinetics across phases of the menstrual cycle in healthy, nonsmoking women who are not using oral contraceptives (Kamimori et al., 1999). Decreased paraxanthine or caffeine metabolic rates in healthy postmenopausal women on estrogen replacement therapy suggest that exogenous estrogen in older women may inhibit caffeine metabolism through the P450 isozyme CYP1A2, an isozyme common to both estrogen and caffeine metabolism (Pollock et al., 1999). Additionally, it is known that oral contraceptive use can double caffeine half-life (Abernethy and Todd, 1985; Patwardhan et al., 1980). The effects of newer oral contraceptives on caffeine half-life have not been studied.
PHYSIOLOGICAL EFFECTS
Caffeine administration affects the functioning of the cardiovascular, respiratory, renal, and nervous systems. Proposed mechanisms of action differ for different physiological effects. Caffeine action is thought to be mediated via several mechanisms: the antagonism of adenosine receptors, the inhibition of phosphodiesterase, the release of calcium from intracellular stores, and antagonism of benzodiazepine receptors (Myers et al., 1999).
Caffeine and Adenosine Receptors
The ability of caffeine to inhibit adenosine receptors appears to be highly important in its effects on behavior and cognitive function. This ability results from the competitive binding of caffeine and paraxanthine to adenosine receptors and is of importance in contributing to CNS effects, especially those involving the neuromodulatory effects of adenosine. Due to the blocking of adenosine inhibitory effects through its receptors, caffeine indirectly affects the release of norepinephrine, dopamine, acetylcholine, serotonin, glutamate, gamma-aminobutyric acid (GABA), and perhaps neuropeptides (Daly et al., 1999).
There are two main classes of adenosine receptor: A1 and A2; caffeine and paraxanthine are nonselective antagonists at both, although they are not especially potent antagonists. The caffeine concentrations attained in vivo that cause mild CNS stimulation (5–10 µM) and that are associated with antiasthmatic effects (50 µM), are in the range associated with adenosine receptor blockade (as quantitated by in vitro receptor binding assays) (Daly, 1993).
Caffeine and Phosphodiesterase
Caffeine increases intracellular concentrations of cyclic adenosine monophosphate (cAMP) by inhibiting phosphodiesterase enzymes in skeletal muscle and adipose tissues. These actions promote lipolysis via the activation of hormone-sensitive lipases with the release of free fatty acids and glycerol. The increased availability of these fuels in skeletal muscle acts to spare the consumption of muscle glycogen. Increased cAMP could also lead to an increase in blood catecholamines. However, caffeine is a fairly weak inhibitor of phosphodiesterase enzymes, and the in vivo concentrations at which behavioral effects occur are probably too low to be associated with meaningful phosphodiesterase inhibition (Burg and Werner, 1975; Daly, 1993).
In contrast, phosphodiesterase inhibition may account for caffeine's (and theophylline's) cardiostimulatory and antiasthmatic actions, since nonxanthine phosphodiesterases are cardiac stimulants (Schmitz et al., 1989) and are also effective as bronchiolar and tracheal relaxants. Indeed, in the latter case, the potency correlates with phosphodiesterase inhibition, not with affinity for adenosine receptors (Brackett et al., 1990; Persson et al., 1982; Polson et al., 1985).
Caffeine and Calcium Mobilization
The earliest proposed mechanism of action for caffeine involved the mobilization of intracellular calcium. Certain actions of caffeine in skeletal muscle appear to involve ionic calcium (Ca++). Caffeine in high concentrations (1–10 mM) was found to interfere with the uptake and storage of calcium in the sarcoplasmic reticulum of striated muscle and to increase the translocation of Ca++ through the plasma membrane (Nehlig et al., 1992). Caffeine may also increase myofilamental sensitivity to Ca++ through its binding to ryanodine receptors in calcium channels of muscle and brain (McPherson et al., 1991).
Although caffeine has been shown to release calcium from intracellular storage pools (sarcoplasmic reticulum) in skeletal and cardiac muscle, the threshold concentration required in vitro to observe this effect (250 µM) is substantially higher than the concentrations required in vivo for cardiac stimulation (50 µM). Hence, this subcellular action of caffeine is probably physiologically irrelevant (though it conceivably could be relevant at toxic concentrations of caffeine) (Daly, 1993).
Caffeine and Benzodiazepine Receptors
Caffeine modifies or antagonizes the effects of benzodiazepines on behavior in both animals and humans (de Angelis et al., 1982; ME Mattila et al., 1992; MJ Mattila et al., 1992). The mechanism for this antagonism was proposed to be the blocking of benzodiazepine receptors by caffeine. Caffeine does have weak antagonistic properties at these receptors. However, this mechanism requires very high concentrations of caffeine (Nehlig et al., 1987; Weir and Hruska, 1983). More recent evidence (Lopez et al., 1989; Nehlig et al., 1992) suggests that the interaction between caffeine and benzodiazepines is mediated through caffeine's effects on adenosine receptors. There is some evidence that caffeine may also be a histamine receptor antagonist (Acquaviva et al., 1986).
General Effects of Caffeine on Physiological Functions
The effects of caffeine on sodium-potassium-adenosine triphosphate pump activity lead to a decrease in plasma potassium concentrations, and affect the depolarization-repolarization process during exercise with potential effects on fine motor coordination.
The effects of caffeine on the heart are primarily stimulatory and are accompanied by increased coronary blood flow. These effects are thought to be mediated not by an action on adenosine receptors (Collis et al., 1984), but instead via phosphodiesterase inhibition. In the lungs caffeine can cause smooth muscle relaxation and bronchial dilatation, possibly accounting for its antiasthmatic effects. However, the relative roles of adenosine receptors and phosphodiesterase as mechanisms of caffeine's antiasthmatic actions remain unresolved (Brackett and Daly, 1991; Ghai et al., 1987; Persson et al., 1982).
The effects of caffeine on the kidney—diuresis, increased blood flow, and rennin secretion—appear to be due to an action of caffeine at adenosine receptors (Spielman and Arend, 1991). Caffeine's behavioral effects appear to be mediated both through adenosine receptors and phosphodiesterase effects and can readily be seen on neurochemically specific neurons. Caffeine's stimulatory action on dopamine, norepinephrine, serotonin, acetylcholine, glutamate, and GABA neurons is hypothesized to result from its ability to block the action of adenosine, which typically inhibits neuronal function. Phosphodiesterase inhibition by xanthines may also account for some stimulatory effects.
Interactions with other nutrients and drugs also characterize certain effects attributed to caffeine. Such interactions include those associated with aspirin, alcohol, nicotine, cocaine, certain other botanicals, and other narcotics (Callahan et al., 1982; Falk and Lau, 1991; Kuribara and Tadokoro, 1992; Parsons and Neims, 1978; White, 1999).
The repeated administration of caffeine does not change its pharmacokinetics, but in many cases development of tolerance does occur. Tolerance is not observed for all effects of the drug, such as fat cell lipolysis (Holtzman et al., 1991), but is seen for certain behavioral actions, such as some of its stimulant properties (increase in locomotor activity in rats) (Finn and Holtzman, 1986). Following the cessation of caffeine use, withdrawal-like symptoms are sometimes seen in humans, such as headache, irritability, nervousness, and a reduction in energy (Griffiths et al., 1986, 1990). The physiological bases for these symptoms are not known. Although the development of withdrawal symptoms might indicate an addictive property, caffeine does not have a convincing profile as an addictive drug.
SUMMARY
Caffeine is rapidly and completely absorbed within an hour following ingestion. It is distributed throughout body water and readily crosses cell membranes including the brain. Its primary mechanisms for stimulatory activity appear to be the blocking of adenosine receptors and inhibition of phosphodiesterases. Caffeine is metabolized and excreted in humans primarily as paraxanthine, which also has pharmacologic activity. With repeated caffeine dosing, paraxanthine may contribute to development of tolerance and withdrawal symptoms. Caffeine clearance rates are affected by both environmental and physiological factors, such as use of oral contraceptives, smoking, and pregnancy. Tolerance to some of caffeine's physiological affects develops with continued use.
hunterllostastings.blogspot.com
Source: https://www.ncbi.nlm.nih.gov/books/NBK223808/
0 Response to "What Property of Caffeine Makes It Easy to Extract edu"
Post a Comment